Introduction
Aging leads to functional decline in various organs and systems of the body through a multitude of physiological, anatomical, and immunological changes. The aging process affects the respiratory system, just like it does with other parts of the body. However, while many physicians are familiar with common pulmonary diseases that often occur in elderly patients, there is a lack of awareness regarding the normal physiological changes that occur in patients as a part of aging. The differentiation between the effects of natural aging-related physiological changes and damage caused by external factors, such as chronic exposure to pollutants, recurrent lung infections, smoking, lifestyle habits, occupational environments, and socioeconomic factors, is particularly challenging within the respiratory system, compared to other organ systems.
In this review, we aim to investigate the changes in the aging respiratory system. We will categorize these changes into alterations in pulmonary function, structural anatomical changes, modifications in gas exchange, defense mechanisms, immune system changes, and alterations in exercise capacity. By gaining a precise understanding of how aging affects the respiratory system, we can enhance our comprehension of how to manage and prevent deterioration in respiratory function among elderly patients. This understanding will aid in the development of treatment strategies customized for elderly patients.
Main Text
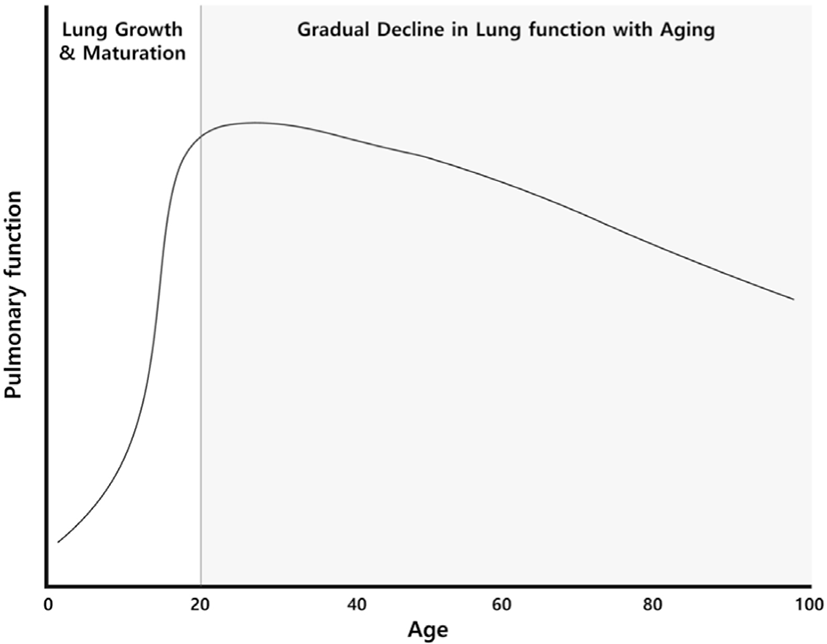
Pulmonary function changes with age. Lung growth and maturation occur over approximately the first 20 years of life, reaching their peak around the age of 25 for males and around 20 for females [1]. After the age of 35, a significant deterioration in pulmonary function becomes increasingly evident (Fig. 1). Factors like smoking, exposure to air pollution, and the presence of respiratory diseases can expedite this rapid decline in pulmonary function [2-4]. Upon closer inspection of age-related changes in pulmonary function, forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) gradually decrease, while residual volume (RV) and functional residual capacity (FRC) increase. Total lung capacity (TLC) remains relatively constant throughout one's lifetime.
As individuals age, FVC decreases due to increased stiffness of the chest wall, loss of elasticity in lung tissue, and weakening of respiratory muscles. There is an average reduction of about 20-25 ml in FVC after the age of 40 [5,6]. FEV1 also decreases, with the rate of decline accelerating with aging. It is reported that the annual decrease in FEV1 is approximately 20-35 ml between the ages of 25-40. When individuals reach the age of 70 or older, the annual decrease is around 40-60 ml [6-10]. Nevertheless, due to substantial individual variability in FEV1, it is difficult to accurately estimate the decline rate because various regression equations are suggested.
It is important to note that the FEV1/FVC ratio decreases with age in both males and females [11] which is why using the FEV1/FVC ratio (<0.7) to define bronchial obstruction may lead to an overdiagnosis of COPD in the elderly [12,13]. There has been a discussion about using a lower limit of normal value, based on the lower 5% of the population, as the diagnostic criterion for COPD to complement this issue. However, the choice of the regression equation for FEV1 greatly influences the lower limit of normal value. Moreover, there is a lack of longitudinal studies using this approach. In light of these considerations, the GOLD guidelines recommend the use of a fixed ratio instead of the lower limit of normal value [1]. This recommendation is based on the understanding that a fixed ratio is not inferior to the lower limit of normal value when it comes to predicting prognosis [14]. Additionally, it is vital to recognize that irreversible airway obstruction is not exclusive to COPD, and clinical presentation and risk factors should be factored in when diagnosing COPD. The simplicity and consistency of diagnosis hold paramount importance in clinical practice. Peak flow rate (PFR) also decreases with age [15] is known that in older individuals, the decreased lung elastic recoil results in increased airway resistance, leading to a reduction in PFR. As for RV and FRC, they tend to increase with age. This is due to a decrease in the elastic recoil of the lungs, which leads to an increase in the size of alveoli. It is known that TLC, which is the sum of FVC and RV, remains relatively constant throughout one's lifetime [5,16].
Chest wall compliance decreases with age. The chest wall comprises the ribs, surrounding muscles, and connective tissue. With aging, calcification and structural changes occur in the ribs and rib joints due to osteoarthritis, causing the chest wall to become rigid and leading to an increase in the anteroposterior diameter at the most expanded position [1]. This condition is known as "barrel chest." Additionally, overinflation of the lungs due to conditions like COPD also influences changes in the chest wall. Kyphosis also contributes to these structural changes. Conditions such as osteoporosis and vertebral fractures induce dorsal kyphosis, leading to a curvature of the thoracic spine that increases the anteroposterior diameter of the chest wall [17]. When chest wall compliance decreases, chest expansion during inhalation is restricted, leading to an increase in the work of breathing. The increased anteroposterior chest wall diameter induces alterations in diaphragmatic curvature and reduces the contractile strength of the diaphragm, ultimately affecting pulmonary function.
The diaphragm is the most vital muscle in the respiratory process, particularly during the inhalation phase. The diaphragm's strength is reported to decline with advancing age. The contractile force of the diaphragm can be measured through transdiaphragmatic pressure (Pdi), which decreases by approximately 25% in older adults compared to young adults [17].
Maximum expiratory pressure (MEP) and maximum inspiratory pressure (MIP), which reflect respiratory muscle strength, also decrease with age [18-20]. MIP and MEP also decrease with age. MIP was reported to decrease annually by an average of 0.8 to 2.7 cmH2 O between the ages of 65 and 85 [17,21]. Respiratory muscle weakness can become more pronounced with age, especially in cases where there is an additional burden on the respiratory muscles due to conditions such as pneumonia or heart failure.
The maximum voluntary ventilation (MVV) also decreases, with a study reporting a 12% decrease in older athletes over a 6-year period [22]. The weakening of respiratory muscle strength with age is attributed to the atrophy of respiratory muscles linked to the aging process, particularly the selective reduction of fast-twitch fibers (Type IIb) [23]. These changes can potentially lead to conditions such as reduced ventilation, decreased exercise tolerance, impaired cough ability, and respiratory failure.
As individuals age, there is a homogeneous degeneration of elastic fibers surrounding alveoli, resulting in excessive dilation of alveolar without the destruction of alveolar walls [24]. This results in an annual decrease in static elastic recoil pressure of 0.1-0.2 cmH2O [25]. Especially, Small airways with a diameter of less than 2 mm close prematurely, causing alveolar overdistention. Chest X-rays of elderly often show uniform hyperlucent lungs with flattened diaphragms, resembling emphysema-like findings. These changes are referred to as senile emphysema, which differs histologically from typical emphysema as it lacks alveolar wall destruction [26]. Understanding these changes can assist in making appropriate interpretations of chest X-rays in the elderly.
Aging reduces the diffusion capacity of the lungs. The diffusing capacity for carbon monoxide (DLCO) decreases annually by 0.2-0.32 ml/min/mmHg in men and 0.06-0.18 ml/min/mmHg in women. This decline becomes more prominent after the age of 40 [6]. The changes in gas exchange due to aging are known to be influenced by imbalances in the ventilation-perfusion ratio, a decrease in alveolar surface area [27], a decrease in pulmonary capillary density [28], and a decrease in blood flow within the pulmonary capillaries [29]. The decline in pulmonary diffusion capacity resulting from age-related alterations in gas exchange can lead to respiratory difficulties.
In the elderly, respiratory diseases tend to exhibit a higher frequency due to impairments in defense mechanisms. Responses to diseases are often diminished, resulting in more severe clinical courses and leaving behind numerous complications. The aging-related impairments in pulmonary defense mechanisms and immunological changes are manifested as follows.
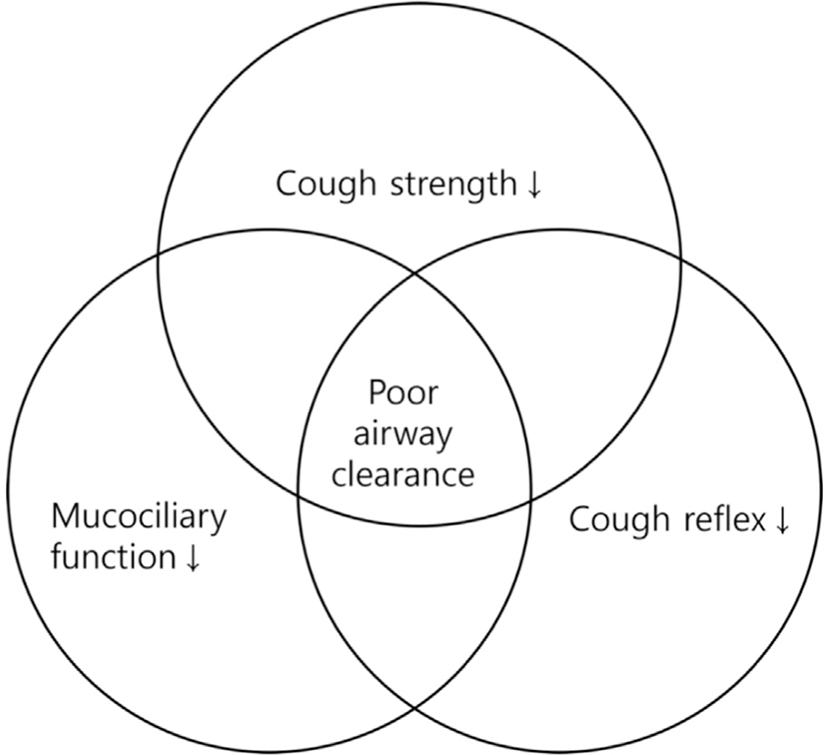
Aging leads to a reduced ability to clear airway secretions, making it more challenging to effectively remove mucus and foreign particles (Fig. 2). As mentioned earlier, the weakening of respiratory muscles due to aging is directly related to a decrease in cough strength. Furthermore, changes in chest and lung compliance reduce the efficiency of coughing. Other contributing factors to the reduced ability to clear airway secretions with age include a decline in cough reflex [30] and impaired ciliary function in the airways [31]. This decline in airway clearance ability can lead to respiratory conditions such as pneumonia, bronchiectasis, and chronic obstructive pulmonary disease.
Minute ventilation is calculated by multiplying tidal volume and respiratory rate. There is no difference in minute ventilation between the elderly and young adults, but the elderly tend to exhibit smaller tidal volumes and higher respiratory rates [32].
Aging significantly reduces responses of heart rate and ventilation to hypoxia (low oxygen levels) and hypercapnia (high carbon dioxide levels) in a resting state. One study reported a 50% reduction in the response to hypoxia and a 40% reduction in the response to hypercapnia in elderly patients compared to younger patients.
Aging is associated with a significant reduction in the heart rate and pulmonary ventilation responses to hypoxia and hypercapnia. One study reported a 50% reduction in the response to hypoxia and a 40% reduction in the response to hypercapnia in elderly patients compared to younger patients [33]. Reduced perception of respiratory abnormalities may be attributed to the diminished efferent neural output in response to hypoxia and hypercapnia [34].
Also, aging impairs the reactivity to increased airway resistance. Airway reactivity is studied using methacholine showed that elderly individuals require a lower dose of methacholine to induce bronchoconstriction [35]. Furthermore, the recovery after treatment with beta-2 agonists takes more time in the elderly [36], and ability to perceive bronchoconstriction are also impaired in the elderly [37]. These changes not only contribute to an increase in asthma-related mortality among the elderly but also reduce awareness of the onset of respiratory diseases such as pneumonia.
When examining bronchoalveolar lavage (BAL) fluid from healthy elderly individuals, researchers observed a decreased proportion of macrophages, increased levels of the immune globulins IgA and IgM, and an elevated CD4+/CD8+ lymphocyte ratio. These findings suggest the presence of activated T-cells in the lower respiratory tract mucosa due to repetitive antigen exposure [38]. Sustained low-grade inflammation in the lower respiratory tract can lead to proteolysis and oxidant-mediated injury in the lung parenchyma, resulting in a decrease in alveoli and impairment of gas exchange through the alveolar membrane.
As people age, exercise capacity generally declines, but the extent of this decline can vary depending on an individual's health and regular physical activity. Maximum oxygen consumption (VO2 max) is known to peak between the ages of 20 and 30 and then gradually decrease by about 1% per year [22]. The rate of this decline can vary based on an individual's level of physical activity, with more significant reductions observed in sedentary adults. Factors contributing to the decline in VO2 max due to aging may include decreased heart rate responsiveness, reduced maximal cardiac output, and a decrease in peripheral muscle mass.
In contrast to the resting state, older individuals are more sensitive to hypercapnia during exercise compared to young adults. A study involving 224 patients aged 56-85 observed that respiratory responses to a constant carbon dioxide production increased with age. [39] This was found to be unrelated to oxygen saturation or metabolic acidosis and is speculated to be due to an increase in dead space ventilation.
Conclusion
The content summarized in the preceding review has been organized in Table 1. These changes in the respiratory system can impair its function in elderly patients, rendering them more vulnerable to various respiratory disorders. This underscores the importance of exercising caution. The global increase in the elderly population is inevitable, and Korean society, in particular, is facing the challenges of becoming an ultra-aged society with a rapidly growing elderly population. Age-related respiratory system changes are a natural physiological process. Understanding and recognizing these changes is crucial not only for preventing and managing respiratory-related diseases in elderly patients but also for enhancing their health and quality of life.